Bioelectronics & Regenerative Engineering.
We have a growing portfolio of cutting-edge biomaterials designed to repair tissues, enhance regeneration and deliver drugs to targeted areas of the body.
Taking our cue from nature, we design environments that mimic those found in the body, in order to influence cells and promote regeneration. We are also developing materials for bioelectronics applications, for neural and cardiac applications.
We are advancing novel manufacturing strategies, such as 3D-printing, remote stimulation and cell patterning, to build scaffolds for cells that recreate the complexity of real tissues.
Scroll down to read more about some examples of our projects.
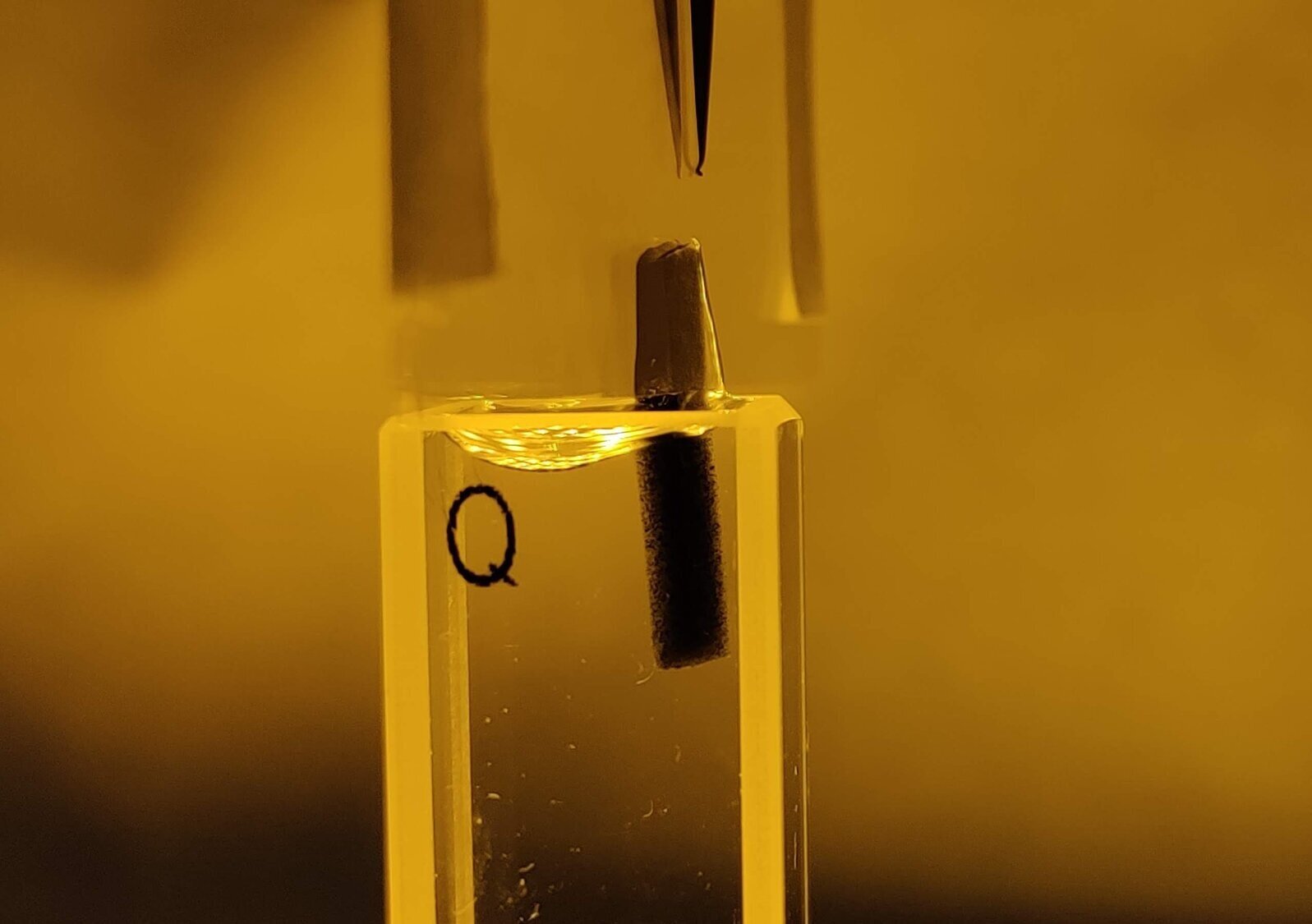
We engineer new ways of triggering gelling in tissue scaffolds.
From gelatin-based sweeties to personal grooming products, gels are familiar every-day materials.
Often we may want to form gels inside the body to create scaffolds that can promote healing. This is commonly achieved using UV light, although the downside with this approach is that it requires additional light-reactive molecules to be placed inside the body.
We have developed a method of creating gels targeted inside specific areas of the body using not light but sound, and not molecules but bubbles. The method involves trapping calcium ions onto the surface of microbubbles (each bubble shown in yellow). Ultrasound (high frequency sound waves) is used to burst the microbubbles, realising the ions and causing a gel to form.
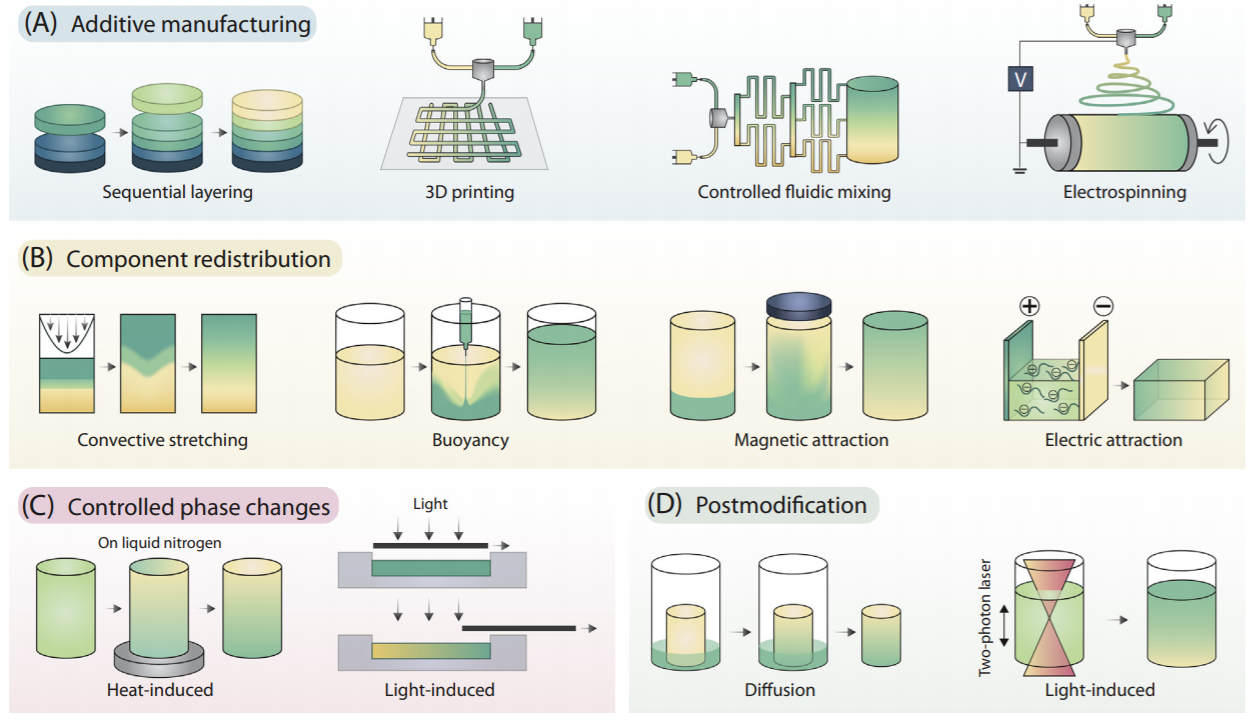
We create gradients with cells and materials to mimic real tissues.
Tissues in the body are rarely made up of a continuous mass of cells. They usually contain gradients in structures and textures. In order for us engineer realistic tissues in the laboratory, we are working on various ways of manipulating cells and materials to achieve gradients.
For example, sound waves can be used to position cells such as myoblasts, which under the right conditions become muscle cells. The sound waves form standing waves - stationary areas of high and low amplitude - and the cells experience a force that moves them towards regions of low amplitude. Changing the frequency of sound and/or the shape of the container allows the formation of complex patterns. Click here to read the paper in Advanced Materials.
A second method for achieving gradients is using buoyancy. Buoyancy - as immortalised Archimedes’ exclamation of “Eureka!” - is the principle whereby an upward force is experienced by materials immersed in a denser liquid, like boats floating on the sea. We use buoyancy to engineer soft materials with gradients of composition, biochemistry and stiffness and pattern these with substances useful in tissue engineering such as nanoparticles, liposomes and proteins. Click here to read the paper in Advanced Materials.
Finally, a third gradient-forming method involves adding magnetic nanoparticles into human heart muscle cells. Applying a magnetic field encourages the cells to align into a pattern typical of muscle tissue. Click here to read the paper in Advanced Materials.
Click here to read our review paper in Trends in Biotechnology.
We research soft robots.
Traditional robots tend to be made out of rigid materials like metal, silicon and plastic, and they carry out a task when instructed to do so by a computer. This is great for simple tasks such as manufacturing, but not so well-suited to devices which interact safely with the human body. This is where soft robots come in.
We develop new materials inspired by nature, which are not only soft, but which don’t rely on motors and mechanics to move; they respond directly to their environment. Our research examines light as a method of controlling soft robots, using a class of materials known as photochromes. These are chemical groups which change their arrangement when we shine a specific colour of light on them. This causes them to change their properties, something which we can reverse when the light goes away. By exploring how to stabilise these molecules in polymers and gels, we hope to develop exciting new ways to control robotic structures.
These materials could find uses in “lab-on-a-chip” systems, cell-driven robotics or in active release drug delivery. The ability to remotely stimulate a robot would be a step-change in how such devices are traditionally controlled. One day it will be possible for soft robots to interact with unfamiliar environments, responding directly to their surroundings in a manner which is closely inspired by nature.
Image credit: Tristan Dell
We are developing a portfolio of 3D scaffold materials for tissue regeneration.
Scaffolds are materials that have been engineered to interact with biological cells and help to form new tissue.
We are continuing to develop a portfolio of scaffolds with a plethora of applications. Their properties range from the ability to self-heal and self-assemble to being bioactive and electroconductive. So far, our scaffolds have been applied to regenerative medicine and stem cell tissue engineering of cardiac tissue, cartilage, collagen-like tissues and bone.
Professor Molly Stevens is the director of the UK Regenerative Medicine Platform’s “Smart Materials Hub”, which aims to develop the next generation of bioactive scaffolds. We are also part of the British Heart Foundation’s Centre for Research Excellence.
Click here to read our review paper in Advanced Functional Materials.
We develop materials and systems for targeted drug delivery, cell reprogramming and vaccines.
Being able to target drugs to a specific area of the body increases the drug’s effectiveness, as well as reducing the dose needed and minimise unpleasant side-effects for the patient. We have developed a portfolio of methods for doing this.
For instance, we encapsulate drugs inside delivery systems such as liposomes and polymersomes; hollow spheres made from fatty molecules and polymers respectively, molecules which are collectively called amphiphiles. We also develop delivery systems based on new amphiphiles, such as ‘cubosomes’. We pre-program these systems by modifying their surfaces, such that they release their cargoes when they detect certain biochemical triggers, such as microRNA, or with physical stimuli such as ultrasound, heat, light or mechanical agitation. Click here to read an example of this work in Advanced Materials. Click here to read our review paper in Advanced Drug Delivery ReviewsClick here to read our paper in Advanced Drug Delivery Reviews.
Many of us are familiar with injections, and few of us enjoy them. However, our work has shown that if you shrink the needle down to the scale of a cell - called a nanoneedle - then cells barely notice their presence at all. We’ve used nanoneedles to inject biological cargo into cells, and even used these nanoneedles as sensors (left). Click here to read an example of this work in Advanced Materials.
In a very exciting recent project, we developed a material that allows delivery of newly developed vaccine technology, self amplifying mRNA, into the body. As well as this, we have pioneered an advance in nanoscale construction that is DNA-based, modular and simple, which enables targeted delivery of therapies. Click here to read the paper in ACS Nano.
As part of the Future Vaccine Manufacturing Research Hub, our work greatly expands the range of therapeutic delivery options, facilitating advances in prevention of acute diseases through vaccine development and treatment of chronic diseases such as cancer.
Selected publications.
C. Li, L. Ouyang, J. P. K. Armstrong and M. M. Stevens
“Advances in the Fabrication of Biomaterials for Gradient Tissue Engineering”
Trends in Biotechnology. 2020. July.
L. Ouyang, J. P. K. Armstrong, M. Salmeron‐Sanchez and M. M. Stevens
“Assembling Living Building Blocks to Engineer Complex Tissues”
Advanced Functional Materials. 2020. 30(26): 1909009.
V. Nele, C. E. Schutt, J. P. Wojciechowski, W. Kit‐Anan, J. J. Doutch, J. P. K. Armstrong and M. M. Stevens
”Ultrasound‐Triggered Enzymatic Gelation.”
Advanced Materials. 2020. 32: 1905914.
A. K. Blakney, Y. Zhu, P. F. McKay, C. R. Bouton, J. Yeow, J. Tang, K. Hu, K. Samnuan, C. L. Grigsby, R. J. Shattock and M. M. Stevens
“Big Is Beautiful: Enhanced saRNA Delivery and Immunogenicity by a Higher Molecular Weight, Bioreducible, Cationic Polymer”
ACS Nano. 2020. 14(5): 5711–5727.
L. Zwi‐Dantsis, B. Wang, C. Marijon, S. Zonetti, A. Ferrini, L. Massi, D. J. Stuckey, C. M. Terracciano and M. M. Stevens
“Remote Magnetic Nanoparticle Manipulation Enables the Dynamic Patterning of Cardiac Tissues”
Advanced Materials. 2020. 32(6): 1904598.
L. Ouyang, J. P. K. Armstrong, Q. Chen, Y. Lin and M. M. Stevens
”Void‐Free 3D Bioprinting for In Situ Endothelialization and Microfluidic Perfusion.”
Advanced Functional Materials. 2019. 30: 1908349.
C. Li, L. Ouyang, I. J. Pence, A. C. Moore, Y. Lin, C. W. Winter, J. P. K. Armstrong and M. M. Stevens
“Buoyancy‐Driven Gradients for Biomaterial Fabrication and Tissue Engineering”
Advanced Materials. 2019. 31(17): 1900291.
U. Kauscher, M. N. Holme, M. Björnmalm and M. M. Stevens
“Physical stimuli-responsive vesicles in drug delivery: Beyond liposomes and polymersomes”
Advanced Drug Delivery Reviews. 2019. 138: 259.
H. M. G. Barriga, M. N. Holme and M. M. Stevens
“Cubosomes: The Next Generation of Smart Lipid Nanoparticles?”
Angewandte Chemie Int. Ed. 2019. 58(10): 2958.
J. P. K. Armstrong, J. L. Puetzer, A. Serio, A. G. Guex, M. Kapnisi, A. Breant, Y. Zong, V. Assal, S. C. Skaalure, O. King, T. Murty, C. Meinert, A. C. Franklin, P. G. Bassindale, M. K. Nichols, C. M. Terracciano, D. W. Hutmacher, B. W. Drinkwater, T. J. Klein, A. W. Perriman and M. M. Stevens
”Engineering Anisotropic Muscle Tissue using Acoustic Cell Patterning.”
Advanced Materials. 2018. 30(43): 1802649.
M. Kapnisi, C. Mansfield, C. Marijon, A. G. Guex, F. Perbellini, I. Bardi, E. J. Humphrey, J. L. Puetzer, D. Mawad, D. C. Koutsogeorgis, D. J. Stuckey, C. M. Terracciano, S. E. Harding and M. M. Stevens
“Auxetic Cardiac Patches with Tunable Mechanical and Conductive Properties toward Treating Myocardial Infarction”
Advanced Functional Materials. 2018. 28(21): 1800618.
C. Horejs, J.-P. St-Pierre, J. R. M. Ojala, J. A. M. Steele, P. B. da Silva, A. Rynne-Vidal, S. A. Maynard, C. S. Hansel, C. Rodríguez-Fernández, M. M. Mazo, A. Y. F. You, A. J. Wang, T. von Erlach, K. Tryggvason, M. López-Cabrera and M. M. Stevens
"Preventing tissue fibrosis by local biomaterials interfacing of specific cryptic extracellular matrix information."
Nature Communications. 2017. 8: 15509.
E. Kim, L. Zwi-Dantsis, N. Reznikov, C. S. Hansel, S. Agarwal and M. M. Stevens
“One-Pot Synthesis of Multiple Protein-Encapsulated DNA Flowers and Their Application in Intracellular Protein Delivery”
Advanced Materials. 2017. 29(26): 1701086.